Targeted synthesis of natural products with light
Potential pathway for drug development using photoreactions
Advertisement
Photoreactions are driven by light energy and are vital to the synthesis of many natural substances. Since many of these substances are also useful as active medical agents, chemists try to produce them synthetically. But in most cases only one of the possible products has the right spatial structure to make it effective. Researchers at the Technische Universitaet Muenchen (TUM) have now developed a methodology for one of these photoreactions that allows them to produce only the specific molecular variant desired.
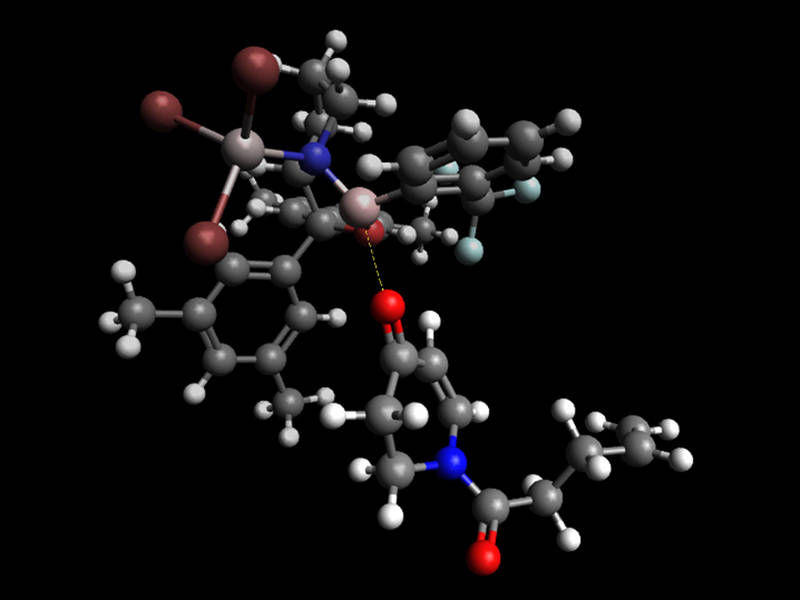
The bulky Lewis acid (above) shields one side of the substrate (bottom) pushing the photoreaction in to the direction of the desired product.
Richard Brimioulle / TUM
For chemists, natural substances are compounds formed by organisms to fulfill the myriad biological functions. This biological activity makes them very interesting for industrial applications, for example as active agents in medication or as plant protection agents. However, since many natural substances are difficult to extract from nature, chemists are working on creating these substances in their laboratories.
A key criterion in the manufacture of natural substances is that they can be produced with the desired spatial configuration. But photoreactions often create two mirror-image variants of the target molecule that can have very different properties. Since only one of theses molecules shows the desired effect, researchers would like to avoid producing the other.
A special catalyst
Thorsten Bach, professor for Organic Chemistry, and his doctoral student Richard Brimioulle have discovered a particularly elegant way of doing this. Their trick was to add a small amount of an electron-deficient compound, a so-called Lewis acid, as a catalyst. The bulky catalyst has a specific spatial structure and forms a complex with the starting substance.
What makes this reaction so special is that the complex of Lewis acid and substrate requires a lower excitation energy than the substrate alone. “Radiating the substance with light of this wavelength favors the formation of the desired substance,” says Richard Brimioulle. “The energy is not sufficient for the non-specific reaction of the uncomplexed substrate.” A further advantage of the synthesis: The Lewis acid is released upon formation of the product and can react with the next molecule of the starting substance. In addition, the reaction takes place in a single step – an important criterion for subsequent industrial deployment.
Elegant pathway to natural substances
Applying photoreactions to the production of natural substances has been a long aspired goal of the scientists headed byProfessor Bach. Using this kind of reaction, even unusually complicated molecular frameworks can be produced quickly and efficiently from simple starting materials. One such molecule is grandisol, a pheromone of the cotton boll weevil. It is already being used as a plant protection agent. Many other agents that inhibit the growth of cancer cells or kill bacteria contain similar kinds of structures and could thus be suitable as medication.
Since other substrates also exhibit reduced excitation energies in the presence of Lewis acids, Bach and Brimioulle suspect that the new method can be used to synthesize many different substances selectively. In future work, the researchers plan to apply the catalysts to other types of photoreactions to give this type of reaction a fixed position among the synthesis methods of organic chemistry.
The research was made possible with funding by the German Research Foundation (DFG) and the Chemical Industry Fund.