New technology detects antibiotic resistance in minutes
Fraunhofer researchers develop innovative diagnostics for detecting antibiotic resistance in bacterial infections
Advertisement
The rapid increase in antibiotic-resistant bacteria is one of the most pressing challenges facing global health. A new transatlantic Fraunhofer research project aims to counter these threats with an innovative diagnostic approach: a microfluidic rapid test system using carbon nanotubes (SWCNT) will make bacterial resistance visible in just a few minutes, significantly faster than conventional methods.
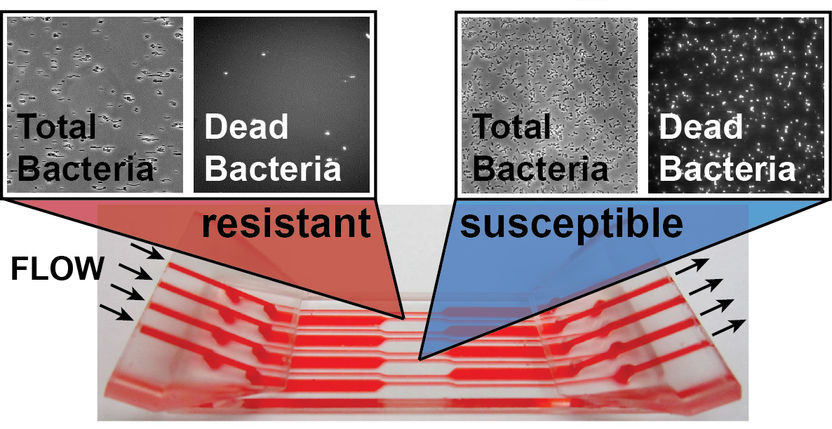
With the new method for rapid antibiotic susceptibility testing (AST), the effect of an antibiotic can be determined in just a few minutes. Instead of waiting 8 to 18 hours for bacterial growth, as was previously the case, it is now possible to observe in real time whether the bacteria die quickly under the influence of antibiotics and flow stress, a clear sign of effectiveness.
© Fraunhofer USA CMI
The widespread and indiscriminate use of antibiotics has led to the rapid spread of microbial resistance to antibiotics. Antibiotic-resistant germs cause around 700,000 deaths worldwide every year. In many of these cases, patients were treated with ineffective antibiotics because the resistance of the pathogens was detected too late.
A transatlantic Fraunhofer research project is now taking a new approach to develop a diagnostic platform that can quickly and easily detect whether a patient is infected with resistant bacteria. The project is being led by the Fraunhofer USA Center for Manufacturing Innovation (CMI) based in Boston. Together with the Fraunhofer Institute for Microelectronic Circuits and Systems IMS (Duisburg) and the Fraunhofer Institute for Interfacial and Bioengineering IGB (Stuttgart), a novel platform for rapid phenotypic antibiotic resistance testing (AST) will be developed over the next 18 months.
An AST tool that can keep pace with the speed of infection
»Clinical microbiology urgently needs flexible and fast AST tools that can keep pace with the speed of infection,« explains Jan Stegemann, project manager at Fraunhofer IMS. »Our goal is to develop a functional platform that can detect metabolic changes as a cellular stress response to an antibiotic well before the pathogen dies, thereby supporting early, evidence-based treatment decisions.«
Currently, many commercially available systems are based on cell growth in microtiter plates and require eight to 16 hours to produce results. The new µFLOWDx platform is expected to deliver usable findings in just a few minutes, potentially revolutionizing research, clinical practice, and pharmaceuticals.
The interdisciplinary project team aims to achieve this with an innovative solution. To enable rapid detection, the bacteria originating from the patient must first be bound to the measuring cell. To this end, the microfluidic system of the diagnostic platform is equipped with special surfaces that exhibit high adhesion to the pathogens. The system also contains highly sensitive optical sensors based on fluorescent carbon nanotubes. If molecules that indicate cellular stress, such as ATP or reactive oxygen species (H₂O₂), are released in response to an antibiotic, the sensors change their fluorescence. This allows microbial resistance to be investigated in real time by flooding the adhered patient bacteria with typical antibiotics: if the pathogen is resistant to the antibiotic tested, the fluorescence signal of the sensor does not change.
Interdisciplinary project team
The project partners are contributing their different areas of expertise to achieve the desired goal: To achieve high bacterial adhesion to the surface of the microfluidics, Fraunhofer IGB is developing suitable surface modifications. For the detection of cellular »stress« molecules, Fraunhofer IMS is contributing its expertise in the development of sensitive optical biosensors based on carbon nanotubes. Both components will be integrated into the microfluidic platform by Fraunhofer USA CMI.
The project aims to produce a functional prototype that can be validated under practical conditions. Commercialization as a diagnostic tool is also planned. Initial market analyses show a high level of interest: The clinical microbiology segment is growing and dominated by leading international companies, but there are no solutions that offer comparable speed and sensitivity.
The project is funded as part of Fraunhofer's internal PACT program (grant number 40-11763) to strengthen transatlantic research. It will run from July 1, 2025, to December 31, 2026. In addition to the desired technological developments, joint publications, trade fair appearances, and follow-up applications for international funding programs are also on the agenda.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Diagnostics
Diagnostics is at the heart of modern medicine and forms a crucial interface between research and patient care in the biotech and pharmaceutical industries. It not only enables early detection and monitoring of disease, but also plays a central role in individualized medicine by enabling targeted therapies based on an individual's genetic and molecular signature.

Topic world Diagnostics
Diagnostics is at the heart of modern medicine and forms a crucial interface between research and patient care in the biotech and pharmaceutical industries. It not only enables early detection and monitoring of disease, but also plays a central role in individualized medicine by enabling targeted therapies based on an individual's genetic and molecular signature.