DNA analysis reveals risk of post-meal insulin resistance
Targeted intervention may be possible
Advertisement
Scientists from the Berlin Institute of Health at Charité (BIH), along with colleagues from the United Kingdom, have found clues as to how we maintain constant blood sugar levels after we have eaten. Analyzing the DNA of nearly 55,000 participants from different studies, they identified ten genomic regions containing genetic variants responsible for regulating blood sugar levels after a meal. In further experiments, they were able to show how these genetic variants influence insulin resistance in cells. Their findings, which are now being published in the journal Nature Genetics, may have implications for how we treat type 2 diabetes.
The risk of developing type 2 diabetes increases with age and the degree of obesity, but also due to lack of exercise and genetic predisposition. If left untreated, type 2 diabetes causes nerve and blood vessel problems, which can lead to eye and foot complications and to an increased risk of heart attack and stroke.
The molecule that plays the most important role in the condition is insulin. People who have type 2 diabetes are unable to correctly regulate their blood sugar levels. This is either because their pancreas doesn’t produce enough insulin when blood sugar levels increase, or because their cells are less responsive to insulin, a state known as “insulin resistance.”
Insulin acts on muscle and fat tissues after meals
“Most studies of insulin resistance have looked at fasting subjects several hours after their last meal,” says the leader of this work, Professor Claudia Langenberg, who heads the Computational Medicine Group at the BIH and is also director of the newly established Precision Healthcare University Research Institute (PHURI) at Queen Mary University of London. “During this time, insulin is largely acting on the liver. But we spend most of our time not fasting but in the post-meal period, when insulin acts on our muscle and fat tissues.” Yet little is known about this process, despite it being believed that the molecular mechanisms underlying postprandial, or post-meal, insulin resistance play a major role in the development of type 2 diabetes.
Professor Sir Stephen O’Rahilly, the co-director of the Wellcome-MRC Institute of Metabolic Science at the University of Cambridge, who was also involved in the study, says: “We know there are some people with specific rare genetic disorders in whom insulin works completely normally in the fasting state, where it’s acting mostly on the liver, but very poorly after a meal, when it’s acting mostly on muscle and fat. What has not been clear is whether this sort of problem occurs more commonly in the wider population, and whether it’s relevant to the risk of getting type 2 diabetes.”
DNA analysis of subjects from 28 studies
To shed light on these issues, the international team used genetic data from 28 studies, encompassing more than 55,000 participants, to look for key genetic variants that influenced insulin levels measured two hours after a sugary drink.
The scientists identified 10 new loci – regions of the genome – associated with insulin resistance after the sugary drink. Eight of these regions had already caught the researchers’ attention in previous studies because they were linked to a higher risk of type 2 diabetes.
Glucose transporter brings blood sugar into cells
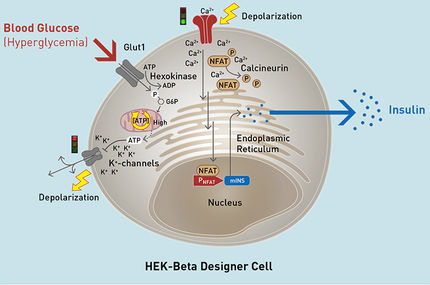
One of these newly-identified loci was located within a gene called GLUT4. This is the gene for a transport protein in the cell membrane of fat and muscle cells that is responsible for moving blood sugar – glucose – into the cells. This locus caused GLUT4 to be less active in the muscle cells.
Further experiments looked at fat cells from mice. Here the scientists turned off individual genes from the ten new loci and observed the effects. “We found 14 different genes that all play a role in glucose transport,” reports Langenberg. “They influence the amount of the glucose transporter GLUT4 found on the surface of the cells. The less GLUT4 that makes it way to the surface of the cell, the poorer the cell’s ability to uptake glucose from the blood.”
Targeted intervention may be possible
Langenberg is hopeful that this discovery will lead to new avenues for preventing type 2 diabetes: “Our work shows how combining dynamic metabolic tests in large numbers of subjects with genetic information can provide important medical insights. We now better understand how blood glucose levels are regulated after a meal, and that opens up the potential for targeted interventions.”