Cre-Controlled CRISPR: conditional gene inactivation just got easier
Advertisement
The ability to turn a gene off only in a specific cell type is essential to modern life science. Thanks to the Cre-Controlled CRISPR it has just became simpler. The new method developed by researchers from the Center for Regenerative Therapies Dresden (CRTD) at TU Dresden with support from the DRESDEN-concept Genome Center (DCGC) offers a fast and easy approach for conditional gene inactivation.
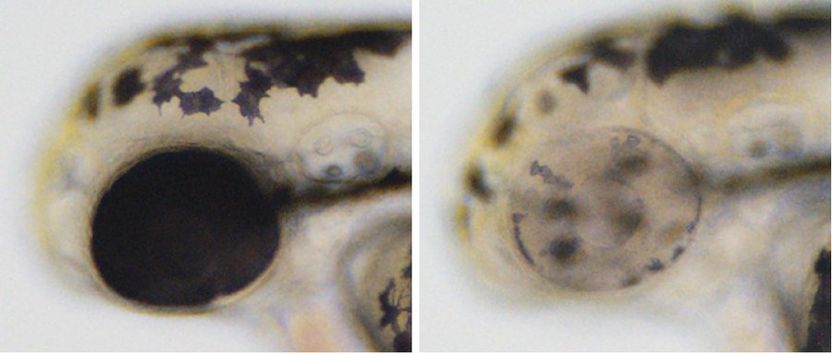
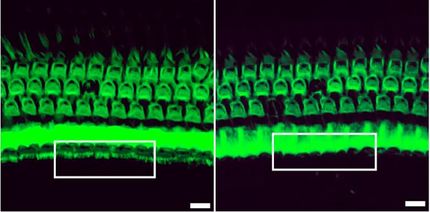
Cre-Controlled CRISPR mutagenesis at work. Left: wild-type sibling with black pigment in melanocytes along the body and in the developing eye. Right: tissue-specific gene inactivation resulting in loss of pigmentation in the developing eye but not in the melanocytes along the body.
© Stefan Hans
To discover the function of a gene researchers turn it off and observe the consequences. Often genes have multiple functions that differ depending on a tissue and age. Some genes are essential to growth and turning them off too early can have profound consequences that can make observing other functions impossible. To avoid it, researchers have been using conditional gene inactivation which allows turning a gene off only in a specific tissue or later in development, e.g., in adulthood.
One of the systems used for conditional gene inactivation is Cre/lox. “It is the gold standard for the conditional gene inactivation in mice but over time has also become quite important in other model organisms such as zebrafish,” says Dr. Stefan Hans, researcher at the CRTD and author of the study. The Cre/lox system relies on an enzyme, known as Cre recombinase, and special sequences, known as lox. The lox sequences are inserted into the genome around the gene of interest. Cre recognizes the lox sequences and removes the gene sandwiched between them. In such a way, the gene is turned off and will not be expressed in this cell. Over the years, the research community developed many lines of animals in which Cre recombinase is only present in a specific tissue. Using such Cre lines, it is possible to turn a gene off only in one tissue but not in the others.
Although commonly used, flanking a gene with lox sequences has its disadvantages. “Inactivating a specific gene takes a lot of time and effort. It requires sophisticated genome modifications and can take multiple generations of animals until the setup is ready for an experiment,” explains Dr. Hans. “Compared to the method we established, it is slow and very laborious,” he adds.
Cre-Controlled CRISPR, the new method developed by a team of Dresden researchers led by Prof. Michael Brand, combines the benefits of the Cre/lox system with the CRISPR/Cas9 genetic scissors. The CRISPR/Cas9 system is a relatively new method that quickly revolutionized life sciences and led to 2020 Nobel Prize in chemistry. Although simple to use in general, CRISPR/Cas9 is not easily limited to just one tissue, which means that adapting it to conditional gene inactivation takes time and effort.
“In Cre-Controlled CRISPR we are taking advantage of the tissue-specific expression of Cre and the ease of gene editing of CRISPR/Cas9,” says Dr. Hans. “By merging the two methods, we created a version of CRISPR/Cas9 that is switched on by a Cre recombinase. Using our method, researchers can still take advantage of vast libraries of already established animal lines that express Cre in different tissues. But Cre-Controlled CRISPR takes away the laborious genome editing because it removes the necessity to flank a gene with lox sequences. It actually requires adding only one sequence to the genome, no matter what the gene of interest is,” explains Dr. Hans. Without the need to sandwich a gene between the lox sequences, Cre-Controlled CRISPR is faster and easier.
The Cre-Controlled CRISPR is not only simpler to establish but, just as CRISPR/Cas9, it also offers the possibility to turn off multiple genes at a time. What is more, the CRTD researchers engineered their method to facilitate subsequent analyses of the cells were Cre-Controlled CRISPR was used. Once the CRISPR/Cas9 is turned on, the cells are labeled with a green fluorescent protein (GFP). Fluorescent proteins such as GFP are commonly used in life science and provide countless methods to separate the labeled cells from others to use them for further experiments, e.g., next generation sequencing or others.
“Although we developed Cre-Controlled CRISPR as a proof of concept in zebrafish, the method is versatile and should be applicable to other model organisms as well,” adds Dr. Hans.
Original publication
Stefan Hans, Daniela Zöller, Juliane Hammer, Johanna Stucke, Sandra Spieß, Gokul Kesavan, Volker Kroehne, Juan Sebastian Eguiguren, Diana Ezhkova, Andreas Petzold, Andreas Dahl and Michael Brand; "Cre-Controlled CRISPR mutagenesis provides fast and easy conditional gene inactivation in zebrafish"; Nature Communications; February 2021