Mapping face sensation in the brainstem
Filippo Rijli and his group at the FMI have shown how the formation of a sensory topographic map in the brainstem is controlled by a single transcription factor, thus shedding light on a decades-old question in neuroscience. The expression of Hoxa2 in brainstem sensory neurons is sufficient to establish a topographic map in which each whisker on the face is represented by a neuronal module (barrelette), permitting accurate mapping of facial sensory input to the brain.
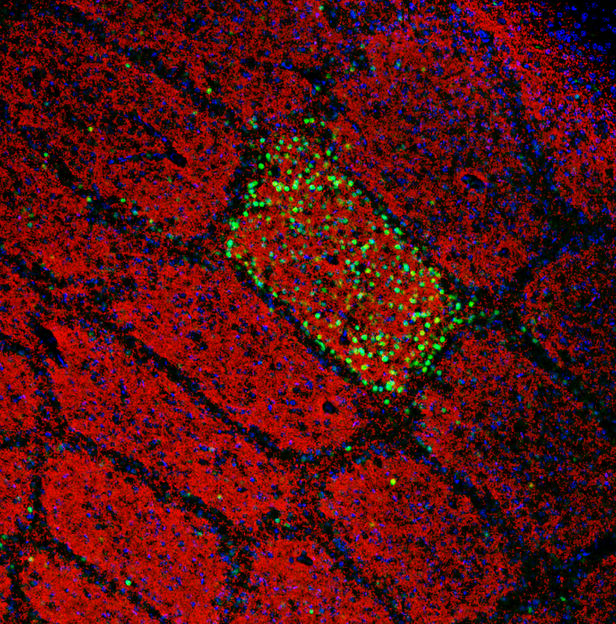
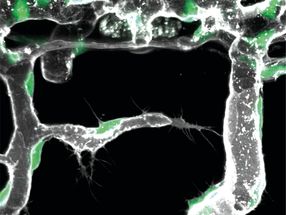
The spatial arrangement of whiskers on the face is maintained in the brain, where whiskers are faithfully mapped as spatially ordered neuronal modules – first in the brainstem (where they are known as barrelettes), then in the thalamus (barreloids) and finally in the cortex (barrels). Red: Barrels in the cortex. Gre Detection of the touch sensation only from the C2 whisker on the face.
Filippo Rijli, FMI
Filippo Rijli and his team at the FMI have now identified a protein that controls the formation of such a map.They describe how the transcription factor Hoxa2 governs the formation of the mouse whisker map in the brainstem.
What fingers are to humans, whiskers are to the mouse. Through the whiskers, the mouse receives tactile information about the size, position and texture of objects in its environment during exploratory behavior. It has been known for about 40 years that a large portion of the somatosensory cortex in rodents is devoted to the representation of whiskers. Moreover, the spatial arrangement of whiskers on the face is maintained in the brain, where whiskers are faithfully mapped as spatially ordered neuronal modules – first in the brainstem, then in the thalamus and finally in the cortex. These whisker maps are serially wired during development, allowing sensation to move from the face to the higher centers in the brain. However, the molecular mechanisms underlying the formation of whisker-related neuronal modules and their spatial organization are still poorly understood.
Ahmad Bechara, a postdoctoral fellow in the Rijli group, has now shown that the presence of Hoxa2 early in development is sufficient by itself to define the area of the somatosensory brainstem targeted by upper jaw axons. Importantly, Hoxa2 is also sufficient to organize the spatial arrangement of barrelettes. When expression of Hoxa2 is forced into an adjacent area of the somatosensory brainstem which usually receives lower jaw axons, a barrelette map is formed in this area at the expense of sensory input from the lower jaw. Rijli explains: “Sensory neurons in the brainstem expressing Hoxa2 are able to attract collaterals from whisker-related afferent axons, and order them topographically such that they can be further refined upon sensory experience into a barrelette map.” They do so by reorienting their dendrites – the branched extensions of nerve cells which make contact with the axon – towards the incoming axons.
Following the neuronal circuitry from the brainstem towards the thalamus, the scientists then showed that the Hoxa2-dependent barrelette map is the necessary template for the barreloid map in the thalamus. “Interestingly,” says Rijli, “Hoxa2 is sufficient to drive topographically directed axon targeting of barrelette neurons to the area of the thalamus that in turn generates the barreloid map. We found that, during development, this thalamic area also received axons relaying information not only from whiskers, but when the whiskers started to send sensory information to the brain via the Hoxa2-expressing neurons in the brainstem, those other connections were pruned so that the barreloid structure remained.”
In a second set of experiments, carried out by Christophe Laumonnerie (a former student in the Rijli group) and Ahmad Bechara, and published in Development, it was additionally shown that the pattern of whiskers on the face is not sufficient to instruct the topographic map in the brain. In a mouse strain with whiskers growing also on the lower jaw, the axons of the peripheral neurons innervating the mandibular whiskers could not organize an additional barrelette pattern in the somatosensory brainstem. These findings nicely complement those described in the Cell Reports paper and support the idea that a whisker-related matching central pattern can only be induced in intrinsically specified neuronal subsets.
Rijli comments: “This is the first time that we have been able to identify a transcriptional mechanism that not only contributes to establish precise connectivity but also helps to build accurate spatial mapping of a sensation from a specific body part into the brain. We hope that this may provide the basis for a better understanding of neurodevelopmental disorders or aberrant sensations after injuries, such as phantom pain.”