Fraunhofer IZI and Novartis Continue Collaboration with CAR-T Cell Therapy
Novartis and Fraunhofer IZI announced that a further agreement has been concluded between Novartis and the Fraunhofer Institute for Cell Therapy and Immunology in Leipzig for the manufacture of the CAR (chimeric antigen receptor)-T cell therapy for patients in Europe over the next few years. The collaboration that began back in 2015 to manufacture CAR-T cell therapies for patients taking part in clinical trials initiated by Novartis is therefore being successfully expanded and continued.
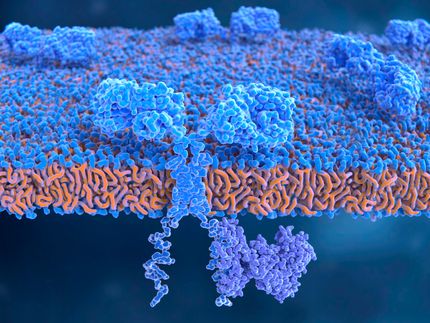
Fraunhofer IZI is a key manufacturing and development site here for the cell therapy Kymriah® (CTL019) provided to patients in Europe taking part in respective clinical trials and Compassionate Use Programs.
On August 27, 2018, Novartis announced that the therapy had been approved by the European approval authority EMA (European Medicines Agency). Over the next few years, prescription-only T-cell therapies will now also be manufactured on an interim basis in the Main Department of GMP Cell and Gene Therapy at Fraunhofer IZI, alongside investigational medicinal products.
At a joint press event, Novartis, Fraunhofer IZI and investigators from Frankfurt and Cologne university hospitals discussed the mode of action, the manufacturing process and their experiences so far from the clinical trials.
Most read news
Organizations
Other news from the department science

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.