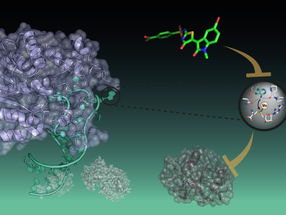
Nanoparticles that could specifically target and kill only cancerous cells
Researchers at the University of Southampton have developed new nanoparticles that can distinguish and penetrate cancerous cells, in a new study which could have major implications for the delivery of anticancer drugs.
The team has designed an advanced type of nanoparticle, which is able to carry drugs directly into cells and release them only in the presence of an appropriate messenger RNA (mRNA), a critical biomolecule for the production of proteins.
As a result, these nanoparticles can identify healthy cells from cancerous cells and release an anticancer drug only to the latter, thus potentially reducing toxicity levels during therapy.
The report marks the first time these nanoparticles can simultaneously detect the presence of two types of mRNA in cells and release two different anticancer drugs to only those cells that express both types of mRNA.
“As far as we know this is the first example of gold nanoparticle dimers that can selectively release two drugs in cancerous cells triggered by specific mRNA signatures," said Dr Antonios Kanaras from the University of Southampton, principal investigator of the research project.
“This is an important step forward for the development of new types of smart nano-technological drugs. The use of smart nanoparticle designs, which release more than one drugs selectively, can ensure minimal damage to healthy cells, be more efficient in smaller doses, and eliminate toxic side effects during the duration of a therapy.”
Nanoparticles are in dimensions about 10,000 times smaller than the thickness of a human hair and new research has revealed that drugs carried using them as a platform can be more concentrated, targeted and more efficient than those delivered through traditional means.
The most important aspect of the nanoparticles presented in this study is that the release of the drugs happens only in the presence of the appropriate messenger mRNAs. The drugs are, therefore, not released in the healthy cells, which do not contain the target mRNA. Such types of smart nanoparticle systems may have the potential to revolutionise nanomedicine and improve patient welfare.
Dr Kanaras added: “Using DNA gold nanoparticle dimers as probes for synergistic mRNA sensing and drug delivery allows to bring together the advantages of having two single nanoparticles into one functional system. For example, this could be translated into knowing the exact number of DNA strands per nanoparticle and therefore control the number of drugs released by each dimer probe. Combining self-assembly nanoparticle strategies and advanced nanoparticle surface chemistry gives more options to design multitasking probes that could be useful in nanomedicine.”
Original publication
Most read news
Original publication
Maria-Eleni Kyriazi, Davide Giust, Afaf H. El-Sagheer, Peter M. Lackie, Otto L. Muskens, Tom Brown, and Antonios G. Kanaras; "Multiplexed mRNA Sensing and Combinatorial-Targeted Drug Delivery Using DNA-Gold Nanoparticle Dimers"; ACS Nano; 2018
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.